Leading Cell Therapy into A New Era
Revolutionizing the treatment of cancer
Simnova and Orna Expand Strategic Partnership to Include BCMA-Targeted RNA Therapeutics
SHANGHAI, CHINA & CAMBRIDGE, Mass. January 15, 2025
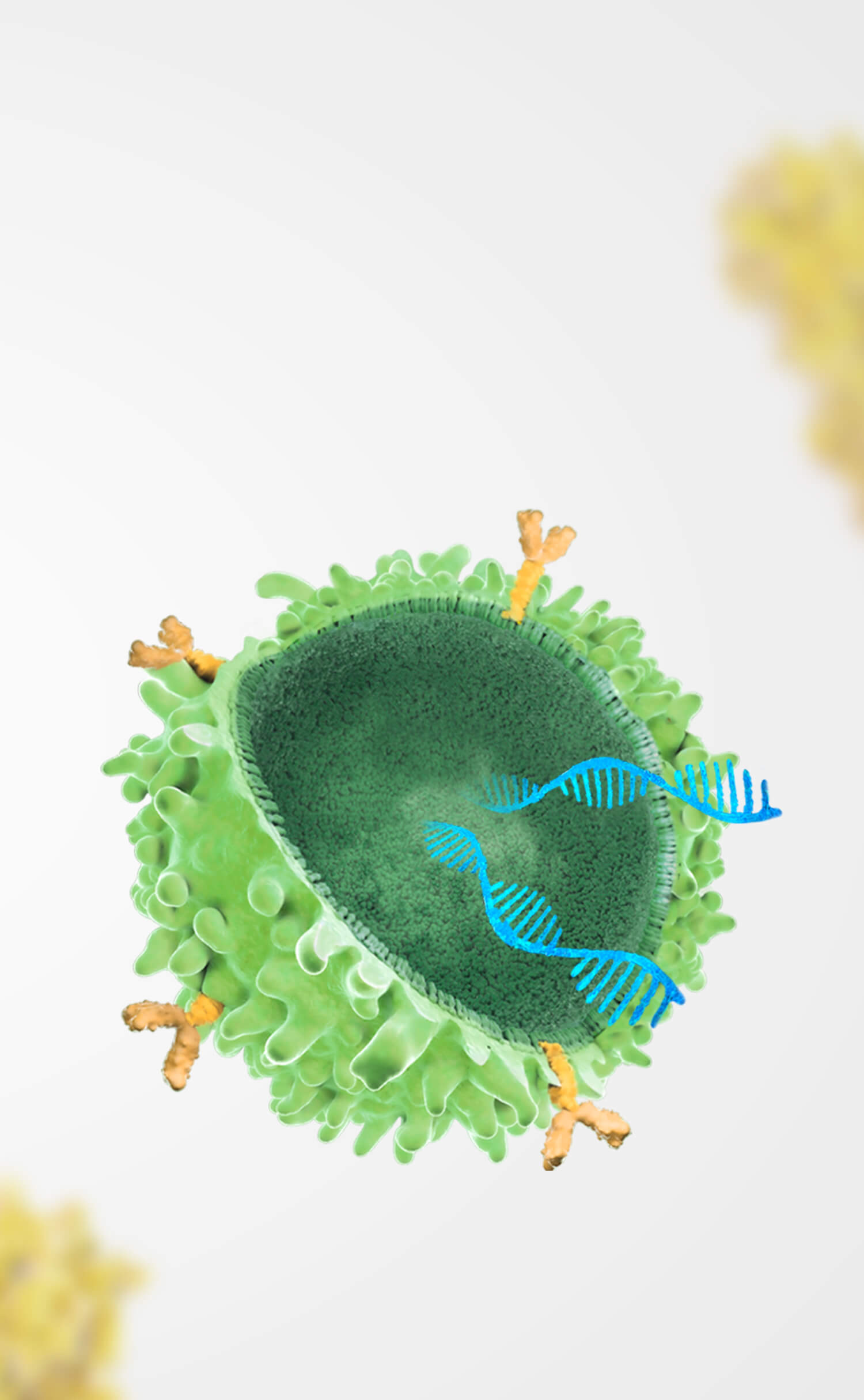
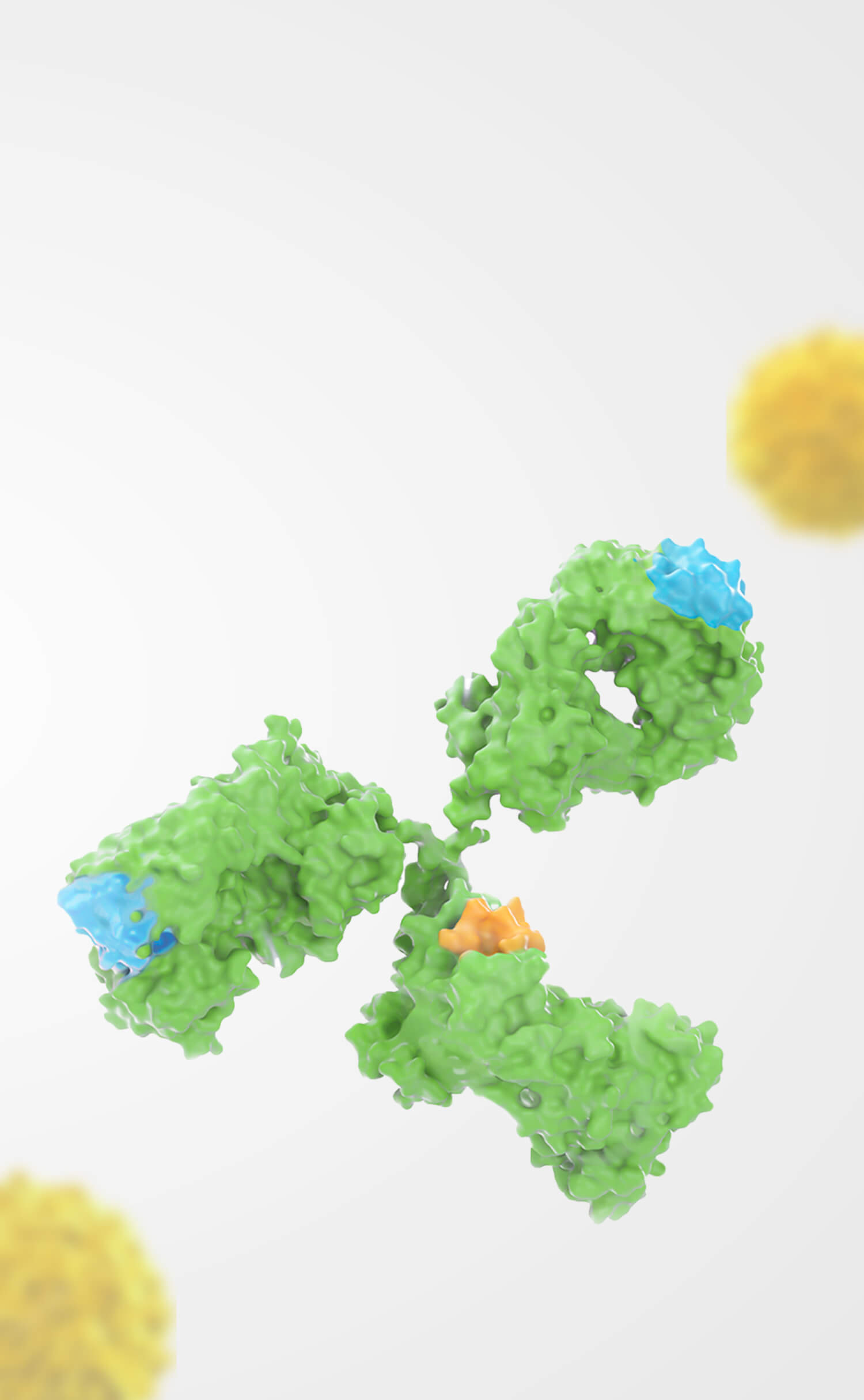
Orna Therapeutics, Inc. ("Orna") and Shanghai Simnova Biotech Co., Ltd. ("Simnova") are pleased to announce the expansion of their strategic collaboration to include BCMA (B-cell maturation antigen) as a designated biological target for RNA-based therapeutics development. This partnership leverages Orna’s groundbreaking circular RNA (oRNA®) technology and Simnova’s expertise in cell therapy to deliver transformative treatments for patients worldwide.
Under the agreement, Simnova will pursue the research and development and commercialization of in vivo panCar cell therapies targeting BCMA in greater China and Orna will retain all rights for development and commercialization in the rest of the world. Each party will have the right to receive an upfront payment within their respective licensed territories and is eligible for clinical development, regulatory, and commercialization milestones as well as royalties on any approved products derived from the collaboration.
In January 2023, Simnova and Orna announced a collaboration agreement granting Simnova exclusive rights to develop and commercialize Orna’s in vivo cell therapy products in the Greater China region. This includes Orna’s lead isCAR project targeting CD19, "ORN-101."
"We are excited to deepen our partnership with Simnova to bring an in vivo BCMA panCAR therapy to patients with multiple Myeloma," said Ansbert Gadicke, M.D., Chairman of Orna and Managing Partner of MPM BioImpact. "Orna’s panCAR approach holds the potential to introduce a novel class of in vivo CAR therapies that overcomes the limitations of current ex vivo cell therapies. The exciting pre-clinical and non-human primate data that Orna has generated continues to reinforce our commitment in this area. We look forward to advancing our programs towards the clinic.”
Dr. Zhuoxiao CAO, CEO of Simnova, added, “Our shared commitment to advancing immunotherapies and oncology treatments opens new opportunities to address unmet medical needs through the development of BCMA-targeted therapeutics.”
About Orna
Orna Therapeutics is dedicated to designing and delivering a new class of fully engineered circular RNA (oRNA®) therapeutics to unlock the potential of RNA medicine to treat diseases anywhere in the body. Orna’s circular RNA transcripts have advantages over traditional mRNA approaches, including simplified production, improved formulation into lipid nanoparticles, and superior protein expression. Its industry-leading LNP-based delivery systems and comprehensive editing programs position Orna to advance novel RNA medicines with vast potential to transform patient care. To learn more, visit www.ornatx.com and follow Orna Therapeutics on X and LinkedIn.
About Simnova
Shanghai Simnova Biotech Co., Ltd. (www.simnovabio.com) is dedicated to bringing innovative cell therapy products to oncology and autoimmune patients. Simnova Biotech has a rich R&D pipeline, with technical advantages in its proprietary universal off-the-shelf CAR-NK and BiTE CAR-T programs, both of which are currently in clinical development stage.

Who we are
Simnova is a clinical stage biotech company focusing on the development of cell therapy for cancer patients.

Our Science
Our innovative NK cell and T cell platforms together with our unique antibody discovery capabilities make these living cell drugs into reality

Our Responsibility
Mission
We are committed to better serving patients and their families and giving life the possibility of choice.
Vision
To develop, produce and commercialize innovative cell and
gene therapies for China and global patients in pursuit of cancer cure.