Therapeutic Focus Area
Our focus is CAR-based cell therapy for cancer patients,
with either hematologic malignancies or solid tumors.
Simnova has a rich R&D pipeline, including both clinical and
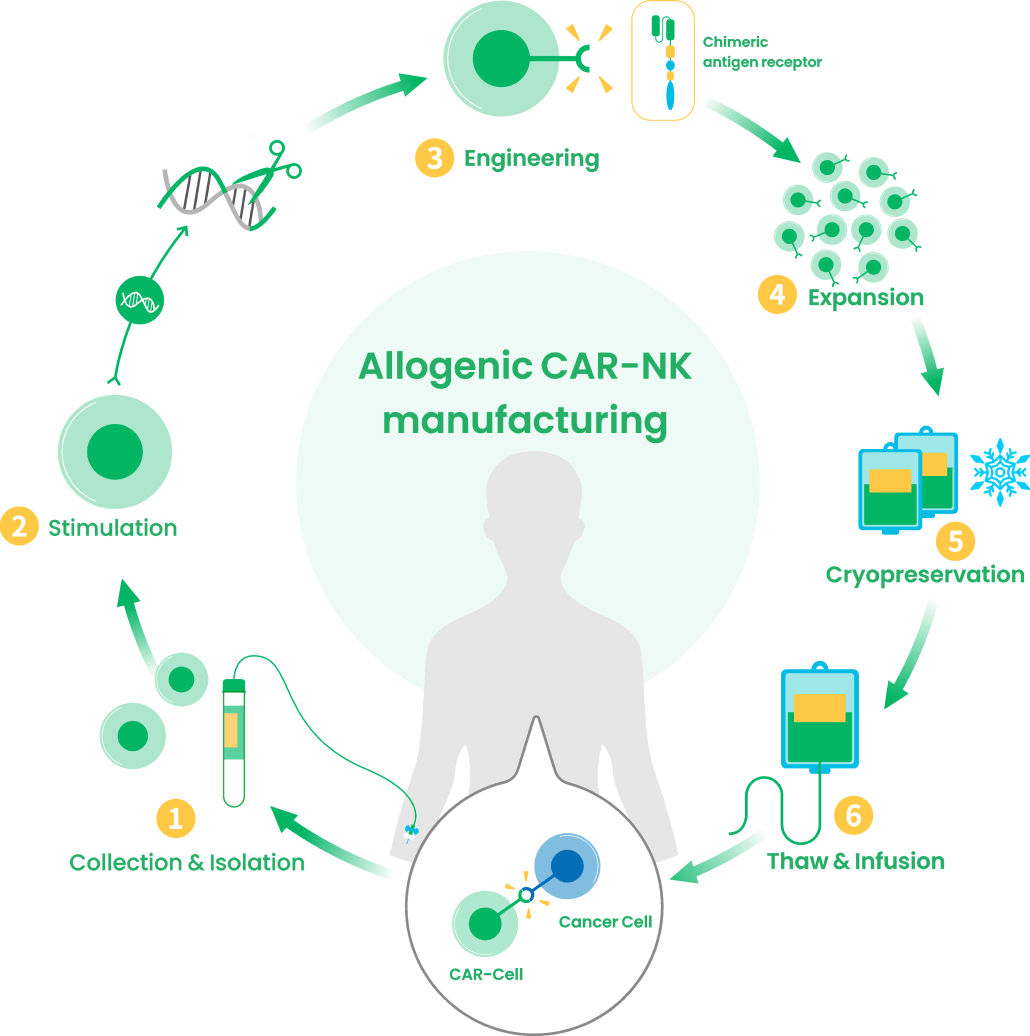
pre-clinical programs. We are developing proprietary off-the-shelf
CAR-NK and BiTE-armed CAR-T cell therapies.
Key Technology Platforms
CMC Capabilities
-
Analytical Development
Product identification, quality purity
- Cell surface marker expression,viability
- Comprehensive flow cytometric-based characterization
Product activity, potency
- Cytokine secretion via ELISA or Bead-based multi cytokine detection
- Number of cells secreting cytokine of interest by ELISPOT
- Luminescence based cytotoxicity assays
- Gene expression, genotyping, copy number
Product stability, safety
- Stability bioassay with product characterization
- Safety testing to include endotoxin, mycoplasma, and bioburden
-
Release Testing
Unprocessed bulk and purified bulk
- Mycoplasma, sterility, product titer, identity, purity, and concentration
- Residual DNA, DNA size, host cell protein, process residual
Final product
- General safety, confirmation of potency, purity, stability, and efficacy
-
Process development and manufacturing
Process development and manufacturing
- We have capabilities to develop a robust, reproducible and scalable manufacturing process
- We have experience handling different cell type, culture system, and gene delivery methods in autologous and allogeneic programs
- Our team are skilled in variety of technology platforms with insight on best practice and scale-up to not only improve safety, but decrease costs of good for patients and industry as a whole
- We have established a CGMP Process Development team to ensure the projects meets NMPA and FDA regulatory requirement with reduced costing going forward and high quality standards
Our Pipeline
| Therapy Type |
Program | Target | Indication | Research | Preclin | IND | PhaseⅠ | PhaseⅡ |
|---|---|---|---|---|---|---|---|---|
| CAR-NK | SNC103 | CD19 | B-cell maligancies | |||||
| SNC112 | BCMA GPRC5D |
Multiple myeloma | ||||||
| SNC113 | Undisclosed | Solid tumor | ||||||
| SNC115 | Undisclosed | Solid tumor | ||||||
| CAR-T | SNC109 | Her2,IL13Rα2 EGFR,EGFRvIII |
Glioblastoma multiforme |
|||||
| SNC102 | BCMA | Multiple myeloma | ||||||
| Program | Research | Preclin | IND | Phase Ⅰ |
Phase Ⅱ |
|---|---|---|---|---|---|
| CAR-NK | |||||
SNC103
|
|||||
SNC112
|
|||||
SNC113
|
|||||
SNC115
|
|||||
| CAR-T | |||||
SNC109
|
|||||
SNC102
|
|||||